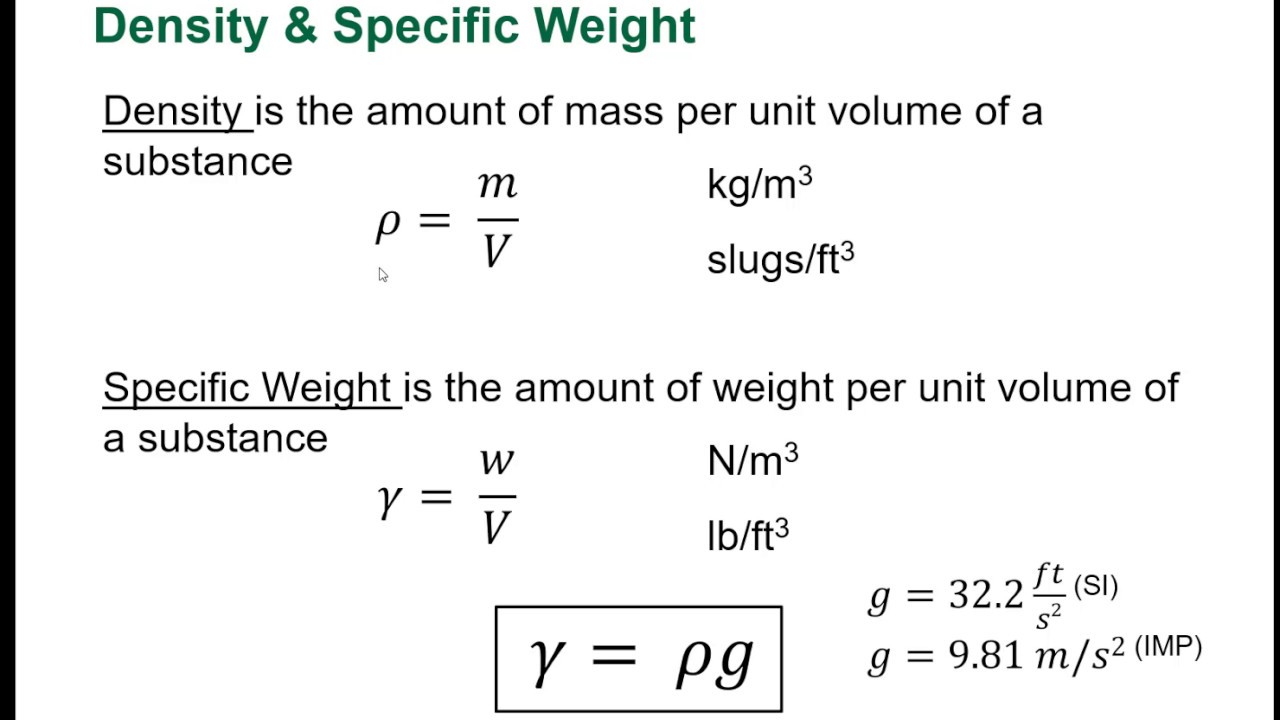Understanding Weight And Density: A Comprehensive Guide
In the realms of physics and engineering, the concepts of weight and density are fundamental to understanding the properties of materials. These two measurements not only define how we perceive mass but also play a crucial role in various applications, from manufacturing to environmental science. This article aims to explore the intricate relationship between weight and density, offering insights into their definitions, calculations, and real-world applications.
Weight is often confused with mass, but it is essential to distinguish between the two. Weight is the force exerted by gravity on an object, while mass refers to the amount of matter in that object. Density, on the other hand, is the mass of an object divided by its volume, providing a measure of how compact or spread out the matter within an object is. Understanding these concepts is not only vital for students and professionals in the sciences but is also applicable in everyday situations.
Throughout this article, we will delve deep into the definitions of weight and density, how they are measured, their formulas, and their significance in various fields. By the end, you will have a comprehensive understanding of these concepts and how they interact with one another.
Table of Contents
- Definition of Weight and Density
- Understanding Weight
- Exploring Density
- The Relationship Between Weight and Density
- Applications of Weight and Density
- Measurement Techniques
- Real-World Examples
- Conclusion
Definition of Weight and Density
Weight is defined as the gravitational force acting on an object. It is calculated using the formula:
- Weight (W) = Mass (m) × Gravitational Acceleration (g)
Where gravitational acceleration (g) is approximately 9.81 m/s² on the surface of the Earth. This means that the weight of an object increases as its mass increases, and vice versa.
Density, in contrast, is defined as the mass of an object divided by its volume. The formula for density is:
- Density (ρ) = Mass (m) / Volume (V)
Where density is typically measured in kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³). Understanding the definitions of these two concepts sets the stage for further exploration.
Understanding Weight
Weight is a force that is dependent on both mass and gravity. It varies depending on the gravitational field strength of the location. For example, an object will weigh less on the Moon than on Earth due to the Moon's weaker gravitational pull.
Calculating Weight
To calculate weight, you need to know the mass of the object and the gravitational acceleration at the location where the object is situated. The formula can be rearranged to find mass if needed:
- Mass (m) = Weight (W) / Gravitational Acceleration (g)
Units of Weight
Weight is commonly expressed in newtons (N) in the SI unit system. However, in everyday scenarios, it is often measured in pounds (lbs) or kilograms (kg).
Exploring Density
Density is a critical property of materials and can provide insights into their structure and composition. Different materials have different densities, which can affect their behavior in various conditions.
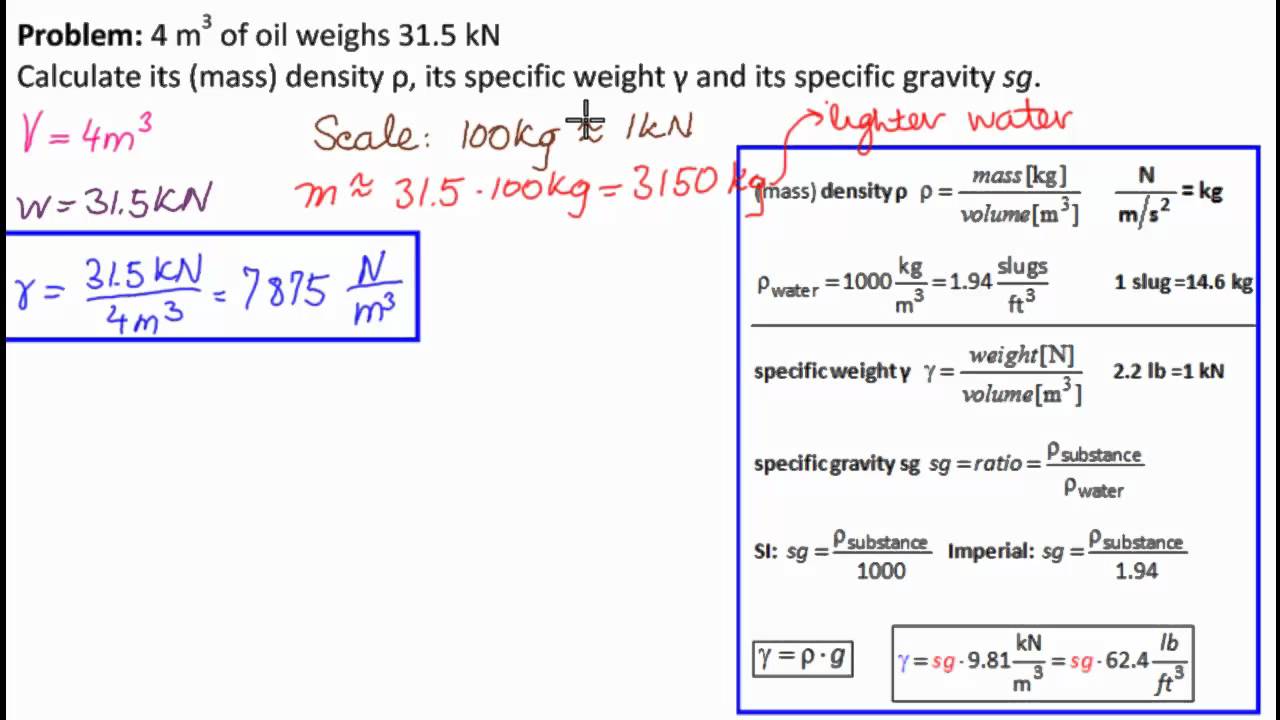
Calculating Density
The calculation of density involves measuring the mass and volume of an object. The volume can be determined through various methods depending on the object's shape:
- For regular shapes, use geometric formulas (e.g., length × width × height).
- For irregular shapes, use water displacement methods.
Units of Density
Density is usually expressed in kg/m³ or g/cm³. For example, the density of water is approximately 1000 kg/m³ or 1 g/cm³, which is a standard reference point in physics.
The Relationship Between Weight and Density
Weight and density are interconnected through the concepts of mass and volume. The density of an object can influence its weight, especially in fluid dynamics where buoyancy plays a role.
When two objects have the same volume, the one with the greater density will weigh more. Conversely, if two objects have the same weight, the one with the greater volume will have a lower density.
Applications of Weight and Density
The principles of weight and density are applied in numerous fields, including:
- Engineering: Designing structures and materials based on their weight-bearing capacity.
- Environmental Science: Understanding buoyancy and sedimentation in bodies of water.
- Manufacturing: Selecting materials based on density for efficiency and cost-effectiveness.
Measurement Techniques
Measuring weight can be done using scales or balances, while density can be measured through methods such as:
- Hydrometer: A device that measures the density of liquids.
- Pycnometer: A laboratory device used for measuring the density of solids and liquids.
Real-World Examples
Understanding weight and density can be illustrated through practical examples:
- Wood vs. Metal: A block of wood and a block of metal of the same size will have different weights due to their differing densities.
- Oil and Water: Oil floats on water due to its lower density, demonstrating the principles of buoyancy.
Conclusion
In conclusion, the concepts of weight and density are essential for comprehending the physical properties of materials. By understanding how to measure and calculate these properties, we can apply this knowledge to various fields and everyday situations.
We encourage you to leave your comments and share this article with others who may benefit from understanding weight and density better. Explore our other articles for more insights into scientific principles!
Sources
- Physics Classroom. (n.d.). Weight and Mass. Retrieved from [source link]
- National Institute of Standards and Technology. (n.d.). Density of Water. Retrieved from [source link]
- Engineering Toolbox. (n.d.). Density of Common Materials. Retrieved from [source link]
Article Recommendations
- 10th Mountain Division
- Michael C Hall
- Zachry
- Fwd
- New York State Education
- Banking Associate
- Who Is Moo Deng
- Wedding Gowns With Gold Embroidery
- Club Random
- Christadler Cvc