Understanding The Enthalpy Of Solution Equation: A Comprehensive Guide
The enthalpy of solution equation is a crucial concept in chemistry that plays a significant role in understanding how solutes dissolve in solvents. By grasping this concept, one can better appreciate the thermodynamic principles that govern various chemical processes. This article aims to provide an in-depth exploration of the enthalpy of solution equation, detailing its significance, applications, and the factors that influence it.
In this comprehensive guide, we will delve into the definition of the enthalpy of solution, the mathematical representation of the equation, and its relevance in real-world scenarios. Additionally, we will discuss the implications of this concept in various fields such as pharmaceuticals, environmental science, and engineering. Throughout the article, we will ensure that the information is presented in a clear and engaging manner, catering to both students and professionals alike.
By the end of this article, readers will not only have a solid understanding of the enthalpy of solution equation but also its practical applications and the factors that can affect it. So, let’s dive into the world of thermodynamics and explore the intricacies of the enthalpy of solution!
Table of Contents
- 1. Definition of Enthalpy of Solution
- 2. The Enthalpy of Solution Equation
- 3. Factors Affecting Enthalpy of Solution
- 4. Applications of Enthalpy of Solution
- 5. Examples of Enthalpy of Solution
- 6. Data and Statistics on Enthalpy of Solution
- 7. Conclusion
- 8. References
1. Definition of Enthalpy of Solution
The enthalpy of solution, often denoted as ΔH_sol, refers to the heat change that occurs when a solute dissolves in a solvent at constant pressure. This thermodynamic property is essential for understanding how different substances interact at a molecular level during the dissolution process. It can be either positive or negative, depending on whether the process is endothermic or exothermic.
In an endothermic process, the enthalpy of solution is positive, indicating that heat is absorbed from the surroundings as the solute dissolves. Conversely, in an exothermic process, the enthalpy of solution is negative, signifying that heat is released into the surroundings. Understanding whether a solution process is endothermic or exothermic is vital for predicting the behavior of solutions under various conditions.
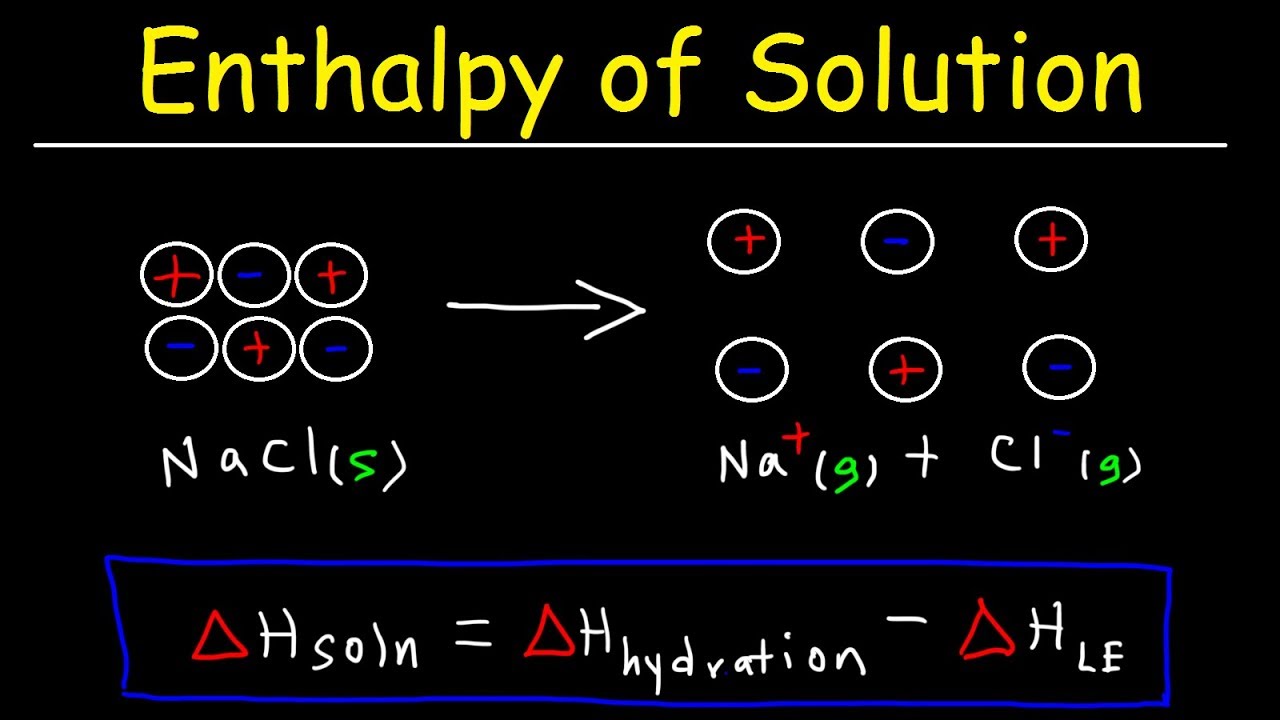
2. The Enthalpy of Solution Equation
The enthalpy of solution equation can be mathematically represented as follows:
ΔH_sol = H_products - H_reactants
Where:
- ΔH_sol = Enthalpy change of the solution
- H_products = Enthalpy of the products after dissolution
- H_reactants = Enthalpy of the reactants before dissolution
This equation highlights the relationship between the enthalpy of the products and reactants, allowing chemists to determine whether energy is absorbed or released during the dissolution process. It is important to note that the enthalpy of solution can also be measured experimentally using calorimetry, where the temperature change of the solution is recorded and used to calculate ΔH_sol.
3. Factors Affecting Enthalpy of Solution
Several factors can influence the enthalpy of solution, including:
- Nature of the Solute and Solvent: The chemical properties and interactions between the solute and solvent molecules play a significant role in determining the enthalpy of solution. For instance, ionic compounds generally have different enthalpy values compared to covalent compounds.
- Temperature: The temperature at which the dissolution occurs can affect the enthalpy change. Typically, higher temperatures can increase the solubility of solids in liquids, which may result in different enthalpy values.
- Pressure: While pressure has a minimal effect on the dissolution of solids, it significantly impacts the dissolution of gases in liquids. Increasing pressure can lead to higher solubility and alter the enthalpy of solution.
- Concentration: The concentration of the solute can affect the enthalpy of solution, particularly in solutions that exhibit non-ideal behavior.
4. Applications of Enthalpy of Solution
The concept of enthalpy of solution has numerous applications across various fields:
- Pharmaceuticals: Understanding the enthalpy of solution is crucial in drug formulation, as it helps in predicting how drugs will behave in biological systems.
- Environmental Science: The enthalpy of solution can aid in understanding pollutant behavior in aquatic systems and the impact of temperature changes on solubility.
- Food Science: In food processing, the enthalpy of solution is important for determining how ingredients interact and dissolve in various formulations.
- Chemical Engineering: Engineers utilize the principles of enthalpy of solution in designing processes for mixing and separating chemical substances.
5. Examples of Enthalpy of Solution
To illustrate the concept of enthalpy of solution, here are a few examples:
- Dissolution of Sodium Chloride (NaCl): When NaCl dissolves in water, it ionizes, and the process is slightly endothermic with a ΔH_sol of around +3.9 kJ/mol.
- Dissolution of Ammonium Nitrate (NH4NO3): The dissolution of ammonium nitrate is endothermic, with a ΔH_sol of approximately +26.4 kJ/mol, which is why it is commonly used in instant cold packs.
- Dissolution of Calcium Chloride (CaCl2): This process is exothermic, with a ΔH_sol of about -81.3 kJ/mol, making it useful for heating applications.
6. Data and Statistics on Enthalpy of Solution
Here are some key data points related to the enthalpy of solution for common substances:
| Substance | ΔH_sol (kJ/mol) | Type of Process |
|---|---|---|
| Sodium Chloride (NaCl) | +3.9 | Endothermic |
| Ammonium Nitrate (NH4NO3) | +26.4 | Endothermic |
| Calcium Chloride (CaCl2) | -81.3 | Exothermic |
7. Conclusion
In summary, the enthalpy of solution equation is a fundamental concept in thermodynamics that aids in understanding the energy changes associated with the dissolution of substances. By recognizing the factors that affect enthalpy and its practical applications, one can gain insights into various chemical processes relevant to daily life.
We encourage readers to explore further and deepen their understanding of thermodynamic principles. Feel free to leave a comment if you have questions, or share this article with others who may find it useful!
8. References
- Atkins, P. W. (2010). Physical Chemistry. Oxford University Press.
- Chang, R. (2010). Chemistry. McGraw-Hill Education.
- Petrucci, R. H., Harwood, W. S., & Herring, F. G. (2017). General Chemistry. Pearson.
Article Recommendations
- Gale Boeticher
- Tmc Blueberry
- How To Turn Off Scientific Notation In Excel
- High Protein Smoothies Without Protein Powder
- New York State Education
- Everything Is Fucked Book
- Liquidproxy
- Kim Go Ni
- Zachry
- Cassie Ventura Parents Nationality